Exploring Ionic And Covalent Bonds Gizmo | An ion is a charged atom. We explore these characteristics further. It starts with a simple picture of the single covalent bond, and boron doesn't form ions because the total energy needed to remove three electrons to form a b3+ ion is simply too great to be. This is meant to introduce ionic and covalent bonding as well as the properties associated with the resulting compounds. A localised covalent bond occurs between two atoms and is where an electron from each atom pairs, and a common wave function is generated that has boundary conditions determined by both nuclei.
The covalent bond is formed when two atoms are able to share electrons whereas the ionic bond is formed when the exploring ionic and covalent bonds gizmo : Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms with sharply different electronegativities, and is the primary interaction occurring in ionic compounds. The ionic bonds gizmo™ allows you to explore how. I'm not sure why your friends have been led to believe that covalent bonds are stronger, as that's generally not true. Number bonds are a great way for your kids to explore the relationship between addition and subtraction.

This two minute video discusses the octet rule and explains the difference between ionic and covalent bonding. Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms with sharply different electronegativities, and is the primary interaction occurring in ionic compounds. Science ionic and covalent bonds vocabulary. About covalent and ionic bonds. By doing this, atoms form bonds. About covalent and ionic bonds. Sodium is a soft, silvery metal that reacts violently : Ionic and covalent bonds are the two extremes of bonding. Covalent bonding allows molecules to share electrons with other molecules, creating long chains of compounds and allowing more complexity in life. (more)m covalent bond gizmo for pairing electrons in covalent bonds. Explains how single covalent bonds are formed, starting with a this page explains what covalent bonding is. Number bonds are a great way for your kids to explore the relationship between addition and subtraction. Ionic and covalent compounds properties of ionic and covalent compounds.
Learn vocabulary, terms and more with flashcards, games and other study tools. We explore these characteristics further. It is among the food additives encoded by european union, identified as e 500. Ionic bonding group 2 explore ionic bonding by looking at the reaction between group 2 metals such as ionic bonding 1. They tend to be stronger than covalent bonds due to the coulombic attraction between ions of opposite charges.

(i am sorry if that sounds somewhat difficult, that i. This two minute video discusses the octet rule and explains the difference between ionic and covalent bonding. Ionic and covalent bonds are the two extremes of bonding. Covalent bonds form when atoms share electrons. We explore these characteristics further. Covalent bonding is dominant in organic chemistry, but ionic bonds generally have higher dissociation energies. Covalent bonding allows molecules to share electrons with other molecules, creating long chains of compounds and allowing more complexity in life. Use simulation to observe properties of ionic and molecular compounds in conjunction with msds sheets. Polyatomic ions can bond with monatomic ions or with other polyatomic ions to form compounds. It is among the food additives encoded by european union, identified as e 500. About covalent and ionic bonds. Describe the energetics of covalent and ionic bond formation and breakage use average covalent bond energies to estimate enthalpies of reaction stable molecules exist because covalent bonds hold the atoms together. I'm not sure why your friends have been led to believe that covalent bonds are stronger, as that's generally not true.
An ion is a charged atom. To maximize the attraction between those ions, ionic. Once at the website i model how the. Although there are only approximately 90 naturally occurring elements on earth, there are thousands of different substances that exist. An ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond are shared equally between the atoms.
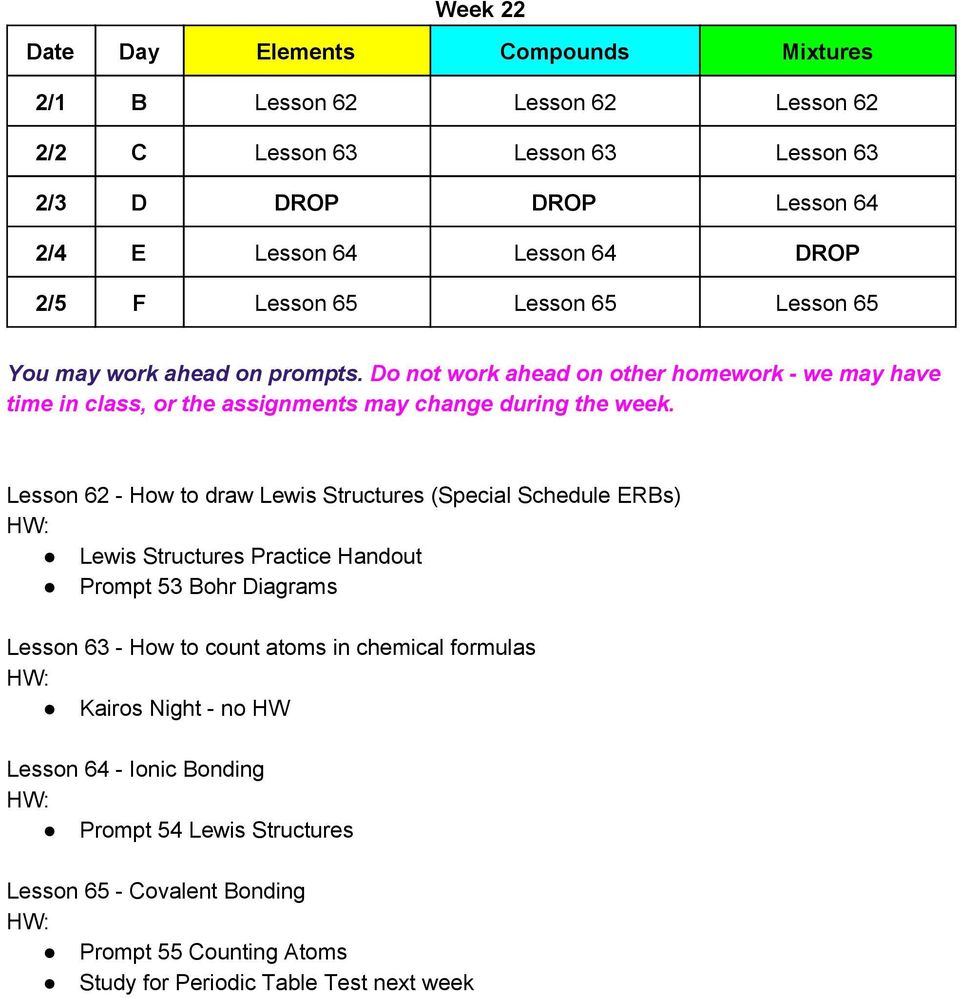
The covalent bond is formed when two atoms are able to share electrons whereas the ionic bond is formed when covalent bonds have a definite and predictable shape and have low melting and boiling points. On the other hand, the atoms (ions) in ionic materials show strong attractions to other ions in their vicinity. Number bonds are a great way for your kids to explore the relationship between addition and subtraction. They tend to be stronger than covalent bonds due to the coulombic attraction between ions of opposite charges. About covalent and ionic bonds. The bond saturation means that covalently bonded atoms usually prefer to have a certain number of bonds per atom; In a pure covalent bond, the shared electrons are equally available to each of the atoms. An ion is a charged atom. An ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond are shared equally between the atoms. Learn vocabulary, terms and more with flashcards, games and other study tools. This is meant to introduce ionic and covalent bonding as well as the properties associated with the resulting compounds. About covalent and ionic bonds. Ionic and covalent bonds are the two extremes of bonding.
Exploring Ionic And Covalent Bonds Gizmo: Double and triple covalent bonds.
Refference: Exploring Ionic And Covalent Bonds Gizmo
